A 74-year-old man who had been self-medicating with antacids for recurrent indigestion consulted his GP when symptoms persisted for three months. After assessment, his GP prescribed a proton pump inhibitor and arranged review in four weeks.
Two months later the patient developed back pain and was prescribed paracetamol with advice about mobilisation. Within six weeks he developed jaundice with dark urine and was admitted to his local hospital. Contrast-enhanced computed tomography confirmed a diagnosis of locally advanced, unresectable pancreatic cancer. He received palliative chemotherapy and died five months later.1
Pancreatic cancer is a deadly disease that mainly affects the elderly (Box 1).2 There will be an estimated 3,271 new cases of pancreatic cancer diagnosed and 2,915 deaths due to pancreatic cancer in Australia in 2017.3 The lifetime risk for pancreatic cancer by age 75 years is 1 in 136 (and 1 in 67 by age 85 years). The average age at diagnosis is 72 years, and two-thirds of patients are aged over 65 years. Pancreatic cancer is rare below age 45 years.4
Outcomes in pancreatic cancer are dismal; currently, the 5-year survival rate is only 8%.1–15 Amongst cancers, only mesothelioma (5-year survival rate of 5%) has a poorer prognosis, while corresponding figures are 98% and 95% for testicular cancer and prostate cancer, respectively.3 The depressingly low 5-year survival for pancreatic cancer has improved from 4% in recent decades, particularly with advances in surgical techniques6 and chemotherapy7,8 (and, more recently, the use of combination chemotherapies), but it is one cancer, along with lung cancer, that has shown only small improvements in outcomes with time.14 In contrast, much greater increases in survival have been achieved for prostate cancer, non-Hodgkin lymphoma, kidney cancer and multiple myeloma.8
Deaths from pancreatic cancer are predicted to steadily increase in men and women (Figure 1), while rates for all other cancers (except lung cancer in women) continue to decrease, making it a priority for finding better preventive and treatment strategies.9,16 Pancreatic cancer is predicted to become the second leading cause of cancer death in the USA by 2030.6,7,9–12
Pancreatic cancer is usually diagnosed too late, at an advanced stage. Over 95% of pancreatic cancers are pancreatic ductal adenocarcinomas. Located in the retroperitoneum of patients who typically present with no or non-specific initial symptoms, the cancer is not diagnosed until it has reached an advanced clinical stage in 80% of patients.4,5,9,13,17 At present, surgical resection is the only potentially curative treatment; but only 15–20% of patients are candidates for pancreatectomy because the majority are diagnosed with disseminated or locally advanced disease.2 Even after surgical resection, most patients have recurrence of their cancer,8 and only 10–20% of patients survive more than 5 years after surgery.18
The complex molecular and genetic makeup of its cancer cells and their surrounding microenvironment contribute to the lethality of pancreatic ductal adenocarcinomas.17 An important aspect of pancreatic cancer is its immunosuppressive tumour microenvironment, which protects it from an immune response that would otherwise clear the tumour.11,12 A hallmark of pancreatic ductal adenocarcinomas is a pronounced fibroinflammatory supportive tissue matrix (stroma), which is pivotal to the processes of tumour formation, progression, invasion, and metastasis.19,20 The stroma has been described as a protective armour of connective tissue, blood vessels and immune cells. The stroma is not just a mechanical barrier. Its cells express multiple proteins which have been associated with a poor prognosis and resistance to treatment.20 Pancreatic tumours also recruit dendritic cells that can switch between preventing or promoting immune responses. Jang et al.19 recently showed that regulatory T cells are responsible for instructing the dendritic cells to prevent anti-tumour immunity. On the other hand, removing the regulatory T cell subset allows dendritic cells to induce a potent anti-tumour immune response.
Apart from age, the greatest risk factor for developing pancreatic cancer is having a strong family history.9,10,14,15 About 10% of cases of pancreatic cancer have a familial basis, and a family history of pancreatic cancer (i.e. having at least 2 affected first-degree relatives or ‘FDRs’ e.g. parents, children, siblings) substantially increases an individual’s risk of developing the disease. The lifetime risk for individuals with 3 or more FDRs is 40%, 10% for 2 FDRs, and 6% for 1 FDR (compared to about 0.7% otherwise).14
The next most well-established risk factor for pancreatic cancer is cigarette smoking, causing a 75% increased risk that persists at least 10 years after smoking cessation.9 Patients with type II diabetes have a 30% excess risk of pancreatic cancer, which persists for more than 20 years after initial diagnosis of diabetes.9 There is also an association between pancreatic cancer and obesity. Several pooled analyses and meta-analyses have reported that obesity increases the risk by 20–50% as compared to people with a normal body mass index.4,9 Excessive alcohol consumption is also a risk factor; heavy drinkers (greater than 8 drinks/day) are more prone to pancreatic cancer.4,14 The incidence of pancreatic cancer and deaths by gender are also slightly higher in males than females (Figure).5
It is an area with conflicting data, but several studies have indicated that statins significantly reduce the risk of developing pancreatic cancer.4,21,22 The benefit may only be in males and could also be more specific to the lipophilic agents (such as atorvastatin, lovastatin and simvastatin) compared with hydrophilic agents (such as pravastatin, rosuvastatin and fluvastatin). In one large case-control study (over 1,400 participants), statin use in men and a duration of statin use of exceeding 10 years were significantly associated with an approximately 50% reduced risk of pancreatic cancer.23 There is perhaps stronger evidence that statins, through a lipidindependent mechanism, can improve survival in patients with diagnosed pancreatic cancer.4,21,24–27
Not only is the pancreas difficult to palpate due to its retroperitoneal location, but there are also no specific blood tests (although that could change soon28) to confirm suspicion of malignancy.17 Endoscopic ultrasonography and endoscopic ultrasonography-guided fine-needle aspiration are currently the preferred means of confirming a diagnosis of pancreatic cancer.5,9 There are no validated early detection strategies for pancreatic cancer, even for high-risk patients.4,14 High-risk populations, such as individuals with a family history, may benefit from surveillance using endoscopic ultrasonography, CT, or MRI.14 For instance, it has been recommended that individuals with at least two FDRs with a history of pancreatic cancer undergo screening by endoscopic ultrasonography and MRI, with further evaluation of any suspicious masses by CT. Surveillance should be performed annually and begin around 50 years of age.14
As alluded to above, a major impediment in successfully treating pancreatic cancer is the late onset of symptoms. Most patients with pancreatic cancer remain asymptomatic until the disease reaches an advanced stage and they generally show metastatic disease by the time of diagnosis. When patients do present, they typically have non-specific symptoms, such as weight loss, anorexia, back or shoulder pain, abdominal pain, nausea and vomiting, dyspepsia, bloating, changes in bowel habit, lethargy, pruritus, and perhaps new-onset diabetes or jaundice.1,8,9,17
The complexities of disease heterogeneity and treatment resistance add to the difficulty of late presentation in contributing to the poor survival of patients with pancreatic cancer.12 Depending on the stage of pancreatic cancer, treatment includes surgery, chemotherapy, radiation therapy, and palliative care. Patients with local disease are evaluated for resection, and offered surgical therapy if they are considered medically fit for pancreatectomy, and the tumour is considered resectable based on available imaging studies (e.g. CT).9,14 The median survival after potentially curative resection resection is only about 18 months and has improved little over the past 30 years. Recurrences typically occur in the retroperitoneum (57%), liver (51%), peritoneum (35%), and lung (15%).14
Adjuvant chemotherapy (with either 5-fluorouracil plus folinic acid, or gemcitabine) has been commonly used after pancreatectomy to reduce the risk of recurrence, in patients who can tolerate chemotherapy. Although gemcitabine has been the drug of choice in this context, largely because of its greater tolerability, the recent ESPAC-4 trial established the combination of gemcitabine and capecitabine as the new treatment of choice in the adjuvant setting after resection for pancreatic ductal adenocarcinoma.7,18 In a multi-centre, randomised trial of gemcitabine alone or in combination with capecitabine for completely resected pancreatic ductal adenocarcinoma, there was a modest and statistically significant improvement in median overall survival, with an absolute increase of 2.5 months for the combination chemotherapy group (28·0 months compared with 25.5 months in the standard gemcitabine group). The 5-year survival rates were 28.8% in the combination group versus 16.3% in the gemcitabine group.7
Patients with locally advanced pancreatic cancer at diagnosis are typically offered chemotherapy, and sometimes chemoradiation, with a goal of reducing disease burden and perhaps undergoing toward resection in the future in a minority of cases (10%).14 Patients with metastatic disease at presentation, who make up the majority of all patients, are offered systemic chemotherapy when they have an appropriate performance status to suggest they will tolerate such treatment.14 Chemotherapy is palliative in this setting, and the principal goals are to control the disease and improve quality of life. Median overall survival for patients with metastatic disease is less than 6 months, but in recent trials, survival has approached 1 year for patients with the highest performance status and who received multi-agent chemotherapeutic regimens.
Either a multi-agent regimen comprising folinic acid, 5-fluorouracil, irinotecan and oxaliplatin (Folfirinox) or gemcitabine plus nanoparticle albumin-bound paclitaxel (nab-paclitaxel) are the treatments of choice for patients who can tolerate these regimens, and are superior to gemcitabine alone in terms of response, progression-free survival, and overall survival in patients with metastatic pancreatic cancer.9,29 Tolerability, however, is a major concern. Folfirinox is associated with an increased risk of febrile neutropaenia, sensory neuropathy, and gastrointestinal toxicities. Therefore, this regimen is recommended only for patients aged 75 years or younger with good performance status.9 The adverse effects of gemcitabine plus nab-paclitaxel seem to be more manageable and this regimen can be used in a wider range of patients. Gemcitabine monotherapy may be indicated in patients with compromised performance status.9
Palliative care is as important as other therapies in pancreatic cancer, because all patients require palliation at some point and the condition is characterised by a high symptom burden at the time of diagnosis and a short survival expectancy. Hence, palliative care plays a key role in the management of these patients.1,2,9 Palliative care can help patients even as they are undergoing treatment for advanced disease.15 Supportive care begins with provision of support and information from the time of diagnosis and the appropriate amount of hope for the patient’s stage of disease.15
The most common complications of pancreatic cancer requiring palliative therapy are due to tumour growth and infiltration of adjacent structures (biliary obstruction, duodenal obstruction, pancreatic insufficiency, pain) and systemic phenomena (cachexia, thromboembolic events).2
More than 75% of patients with pancreatic cancer experience pain due to pancreatic and coeliac plexus infiltration, and adequate treatment is essential to avoid a decrease in quality of life, depression and deterioration of performance status.2 These patients often have severe, epigastric pain radiating to the back either due to direct invasion into the nociceptors in the pancreatic bed or destruction of pancreatic tissue leading to inflammation of the coeliac plexus.8
Potent opioids are typically needed, although caution is required in elderly patients with poor performance status. Another treatment option is a coeliac plexus block. Endoscopic ultrasonography-guided coeliac plexus neurolysis and endoscopic ultrasonography-coeliac ganglion neurolysis are achieved by injection of local anaesthetic (e.g. bupivacaine) followed by absolute alcohol into the coeliac plexus or coeliac ganglia.5 While often effective, the duration of the analgesic effect does not exceed 2–3 months.2
Biliary obstruction is common in patients with pancreatic cancer.2,9 Obstructive jaundice and obstruction of the duodenum in these patients require surgical, endoscopic, or radiological interventions. Endoscopic stenting has become the standard approach in most cases.2,5,9,15 Involvement of a dietitian, along with pancreatic enzyme replacement, may be required to manage the common issues of malnutrition, abdominal bloating, and diarrhoea.5,8,15 Large trials are underway, but the evidence to date indicates that prophylaxis of venous thromboembolism with low molecular-weight heparin is also warranted in patients with advanced pancreatic cancer.15,30,31
Recently published research by Australians from the Garvan Institute of Medical Research in Sydney has generated significant hope that drugs which weaken the protective stroma surrounding pancreatic tumours may improve survival times in sufferers. Timpson and colleagues investigated fasudil, an old vasodilator drug previously used for the treatment of cerebral vasospasm due to subarachnoid haemorrhage. It appears that fasudil can weaken the stroma, making it easier for chemotherapy drugs to enter the tumour. In mice with pancreatic cancer, 3 days of fasudil treatment prior to standard chemotherapy (gemcitabine plus nabpaclitaxel) increased survival time by 47%.32
There is clearly an urgent need for further innovative research that leads to better detection capabilities and to novel drugs with improved efficacy and reduced toxicity in patients with pancreatic cancer.14
Box 1. Pancreatic cancer: key points1,9,15
|
Figure 1. (i) The age-standardised incidence and mortality rate of pancreatic cancer, by sex, since 1982 and (ii) Incidence (2013) and mortality (2014) rates of pancreatic cancer, by age group and sex.3
(i) 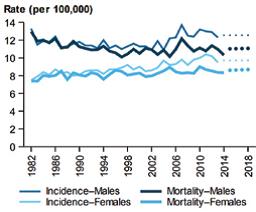 (ii)
(ii) 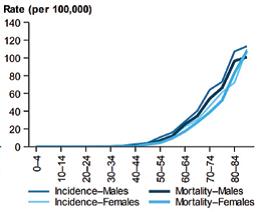
PROFESSOR GREGORY PETERSON MPS is Deputy Dean (Research), Faculty of Health and Co- Director, Health Services Innovation Tasmania, School of Medicine, University of Tasmania and a community pharmacist.
References
-
- Siriwardena AK, Siriwardena AM. Pancreatic cancer. BMJ 2014;349:g6385.
- Higuera O, Ghanem I, Nasimi R, et al. Management of pancreatic cancer in the elderly. World J Gastroenterol 2016;22(2):764–75.
- Australian Institute of Health and Welfare 2017. Cancer in Australia 2017. Cancer series no.101. Cat. no. CAN 100. Canberra: AIHW.
- Kuroczycki-Saniutycz S, Grzeszczuk A, Zwierz ZW, et al. Prevention of pancreatic cancer. Contemp Oncol 2017;21(1):30–4.
- Fogel EL, Shahda S, Sandrasegaran K, et al. A multidisciplinary approach to pancreas cancer in 2016: A Review. Am J Gastroenterol 2017;112(4):537–54.
- Abou-Khalil J, Rocha FG. Surgical strategies and novel therapies for locally advanced pancreatic cancer. J Surg Oncol 2017;116(1):16–24.
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389(10073):1011–24.
- Gupta R, Amanam I, Chung V. Current and future therapies for advanced pancreatic cancer. J Surg Oncol 2017;116(1):25–34.
- Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388(10039):73–85.
- King J, Bouvet M, Singh G, et al. Improving theranostics in pancreatic cancer. J Surg Oncol 2017;116(1):104–13.
- Giovannetti E, van der Borden CL, Frampton AE, et al. Never let it go: Stopping key mechanisms underlying metastasis to fight pancreatic cancer. Semin Cancer Biol 2017;44:43–59.
- Skelton RA, Javed A, Zheng L, et al. Overcoming the resistance of pancreatic cancer to immune checkpoint inhibitors. J Surg Oncol 2017;116(1):55–62.
- Al Efishat M, Wolfgang CL, Weiss MJ. Stage III pancreatic cancer and the role of irreversible electroporation. BMJ 2015;350:h521.
- Yabar CS, Winter JM. Pancreatic cancer: a review. Gastroenterol Clin North Am 2016;45(3):429–45.
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378(9791):607–20.
- Mayor S. Deaths from pancreatic cancer in Europe continue to increase while rates for other cancers fall. BMJ 2014;348:g2914.
- Rossi ML, Rehman AA, Gondi CS. Therapeutic options for the management of pancreatic cancer. World J Gastroenterol 2014;20(32):11142–59.
- Deplanque G, Demartines N. Pancreatic cancer: are more chemotherapy and surgery needed? The Lancet 2017;389(10073):985–86.
- Jang J-E, Hajdu CH, Liot C, et al. Crosstalk between regulatory t cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Reports 2017;20(3):558–71.
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010;362(17):1605–17.
- Gong J, Sachdev E, Robbins LA, et al. Statins and pancreatic cancer. Oncol Lett 2017;13(3):1035–40.
- Carey FJ, Little MW, Pugh TF, et al. The differential effects of statins on the risk of developing pancreatic cancer: a case-control study in two centres in the United Kingdom. Dig Dis Sci 2013;58(11):3308–12.
- Walker EJ, Ko AH, Holly EA, et al. Statin use and risk of pancreatic cancer: results from a large, clinic-based case-control study. Cancer 2015;121(8):1287–94.
- Huang BZ, Chang JI, Li E, et al. Influence of statins and cholesterol on mortality among patients with pancreatic cancer. J Natl Cancer Inst 2017;109(5) doi: 10.1093/jnci/djw275
- Wu BU, Chang J, Jeon CY, et al. Impact of statin use on survival in patients undergoing resection for early-stage pancreatic cancer. Am J Gastroenterol 2015;110(8):1233–9.
- Jeon CY, Pandol SJ, Wu B, et al. The association of statin use after cancer diagnosis with survival in pancreatic cancer patients: a SEER-medicare analysis. PLoS One 2015;10(4):e0121783.
- Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med 2012;367(19):1792–802.
- Kim J, Bamlet WR, Oberg AL, et al. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers. Science Translational Medicine 2017;9
- Kosmidis C, Sapalidis K, Kotidis E, et al. Pancreatic cancer from bench to bedside: molecular pathways and treatment options. Ann Transl Med 2016;4(9):165.
- Bozas G, Muazzam IA, Ilyas W, et al. PO-39 – Primary thromboprophylaxis for ambulatory patients with advanced metastatic pancreatic cancer. A practical implementation of lessons from published experience. Thromb Res 2016;140 Suppl 1:S191.
- Tun NM, Guevara E, Oo TH. Benefit and risk of primary thromboprophylaxis in ambulatory patients with advanced pancreatic cancer receiving chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Blood Coagul Fibrinolysis 2016;27(3):270–4.
Vennin C, Chin VT, Warren SC, et al. Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Science Translational Medicine 2017;9.













 PSA Chief Operating Officer Deb Bowden, Senator Zed Seselja and PSA National President Dr Shane Jackson.[/caption]
PSA Chief Operating Officer Deb Bowden, Senator Zed Seselja and PSA National President Dr Shane Jackson.[/caption]





 [post_title] => New Pharmacy House opens
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => new-pharmacy-house-opens
[to_ping] =>
[pinged] =>
[post_modified] => 2018-04-05 12:33:52
[post_modified_gmt] => 2018-04-05 02:33:52
[post_content_filtered] =>
[post_parent] => 0
[guid] => http://psa.studionerve.com/?p=1231
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[title_attribute] => New Pharmacy House opens
[title] => New Pharmacy House opens
[href] => http://psa.studionerve.com/new-pharmacy-house-opens/
[module_atts:td_module:private] => Array
(
)
[td_review:protected] => Array
(
)
[is_review:protected] =>
[post_thumb_id:protected] => 1239
)
[post_title] => New Pharmacy House opens
[post_excerpt] =>
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => new-pharmacy-house-opens
[to_ping] =>
[pinged] =>
[post_modified] => 2018-04-05 12:33:52
[post_modified_gmt] => 2018-04-05 02:33:52
[post_content_filtered] =>
[post_parent] => 0
[guid] => http://psa.studionerve.com/?p=1231
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[title_attribute] => New Pharmacy House opens
[title] => New Pharmacy House opens
[href] => http://psa.studionerve.com/new-pharmacy-house-opens/
[module_atts:td_module:private] => Array
(
)
[td_review:protected] => Array
(
)
[is_review:protected] =>
[post_thumb_id:protected] => 1239
)